An adverse event following immunization is any untoward medical occurrence (unfavourable or unintended sign, abnormal laboratory finding, symptom or disease) which follows immunization and which does not necessarily have a causal relationship with the usage of the vaccine. Reported adverse events can either be true adverse events - i.e. resulting from the vaccine or immunization process - or coincidental events that are not due to the vaccine or immunization process but are temporally associated with immunization. The five categories of AEFI as defined by CIOMS and WHO are described in table 2.1
Table 2.1 Cause-specific categorization of AEFI (CIOMS/WHO 2012)
Cause-specific type of AEFI | Definition |
Vaccine product-related reaction | An AEFI that is caused or precipitated by a vaccine due to one or more of the inherent properties of the vaccine product. |
Vaccine quality defect-related reaction | An AEFI that is caused or precipitated by a vaccine that is due to one or more quality defects of the vaccine product, including its administration device as provided by the manufacturer. |
Immunization error-related reaction (formerly “programme error") | An AEFI that is caused by inappropriate vaccine handling, prescribing or administration and thus by its nature is preventable. |
Immunization stress related responses (formerly “Immunization anxiety-related reaction" | A range of symptoms and signs that may arise around immunization that are related to “anxiety" or “stress" and not to the vaccine product, a defect in the quality of the vaccine or an error of the immunization programme. They include vasovagal-mediated reactions, hyperventilation-mediated reactions and stress-related psychiatric reactions or disorders.. |
Coincidental event
| An AEFI that is caused by something other than the vaccine product, immunization error or immunization stress, but a temporal association with immunization exists.
|
2.2.1 Vaccine reactions
Based specifically on cause, seriousness and frequency, vaccine reactions may be grouped into two broad categories:
A. Cause-specific vaccine reactions:
• vaccine product-related reaction and
• vaccine quality defect-related reaction
B. Vaccine reactions by seriousness and frequency:
• common or minor reactions;
• rare or serious reactions.
A. Cause-specific vaccine reactions
Vaccine product-related reaction: This is an individual’s reaction to the inherent properties of the vaccine, even when the vaccine has been prepared, handled and administered correctly. Most often the exact mechanism of a vaccine product-related reaction is poorly understood. The reaction may be due to an idiosyncratic immune mediate reaction (e.g. anaphylaxis) or to replication of the vaccine-associated microbial agent (e.g. vaccine-associated poliomyelitis following OPV which contains attenuated live virus).
Vaccine quality defect-related reaction: This is a due to a defect in a vaccine (or its administration device) that occurred during the manufacturing process. Such a defect may have an impact on an individual’s response and thus increase the risk of adverse vaccine reactions. Insufficient inactivation of wild-type vaccine agent (e.g. wild polio virus) during the manufacturing process or contamination introduced during the manufacturing process could cause the vaccine quality defect-related reactions.
B. Vaccine reactions by seriousness and frequency
Most vaccine reactions are minor and subside on their own. Serious reactions are very rare and, in general, do not result in death or long-term disability. Table 2.2 describes the frequency of occurrence of reported adverse events.
Table 2.2 Frequency of occurrence of reported adverse reactions
Frequency category
| Frequency in rate
| Frequency in %
|
Very common | ≥ 1/10 | ≥ 10% |
Common (frequent) | ≥ 1/100 and < 1/10 | ≥ 1% and < 10% |
Uncommon (infrequent) | ≥ 1/1000 and < 1/100 | ≥ 0.1% and < 1% |
Rare | ≥ 1/10 000 and <1/1000 | ≥ 0.01% and < 0.1% |
Very rare | < 1/10 000 | < 0.01%
|
Common, minor vaccine reactions
They are caused when recipient's immune system reacts to antigens or the vaccine's components (e.g. aluminium adjuvant, stabilizers or preservatives) contained in the vaccine. Most AEFI are minor and settle on their own. Minor AEFI could be local or systemic. Local reactions include pain, swelling and redness at injection site. Systemic reactions include fever irritability and malaise. A successful vaccine reduces these reactions to a minimum while producing the best possible immunity. Table 2.3 describes the common minor vaccine reactions by antigen and the treatment for the same.
Table 2.3 Common minor vaccine reactions by antigen and treatment
| Vaccine | Local adverse events (pain, swelling, redness) | Fever (> 380C) | Irritability, malaise and systemic symptoms |
| BCG1 | 90%-95% | - | - |
| Hepatitis B | Adults up to 15% Children up to 5% | 1 – 6% | - |
| Hib | 5-15% | 2%-10% | |
| Measles/MR/MMR | ~10% | 5%-15% | 5% (Rash) |
| OPV | None | Less than 1% | Less than 1%2 |
| Pertussis (DTwP)3 | up to 50% | up to 50% | up to 55% |
| †Pneumococcal conjugate | ~20% | ~20% | ~20% |
| Tetanus/DT/aTd | ~ 10%4 | ~ 10% | ~ 25% |
| Treatment | Cold cloth at injection site and Paracetamol* | Give extra oral fluids, wear cool clothing, tepid sponge or bath and Paracetamol* | Supportive treatment
|
1 Local reactogenicity varies from one vaccine brand to another, depending on the strain and the number of viable antigen in the vaccine.2 Diarrhoea, Headache and/or muscle pains
3 When compared with whole cell pertussis (DTwP) vaccine, acellular pertussis (DTaP) vaccine rates are lower.
4 Rate of local reactions are likely to increase with booster doses, up to 50 -85%.
* Paracetamol dose: up to 15mg/kg every 6-8 hours, maximum of 4 doses in 24 hours
† Source: http://www.cdc.gov/vaccines/pubs/ACIP-list.htm
Rare, more severe (and serious) vaccine reactions
They are caused by the body's reaction to a particular component in a vaccine. The term “severe” is used to describe the intensity of a specific event (as in mild, moderate or severe); the event itself, however, may be of relatively minor medical significance. Severe AEFI can be disabling but are rarely life threatening. Some examples are seizures, thrombocytopenia, Hypotonic Hyporesponsive Episodes (HHE), prolonged crying etc.
Severe AEFI are considered serious by definition if they:
• Result in death
• are life-threatening
• require in-patient hospitalization or prolongation of existing hospitalization
• result in persistent or significant disability/incapacity
• are a congenital anomaly/birth defect
ALL serious AEFI should be reported, investigated and the causality assessed.
The rate of occurrence of the rare and more serious reactions has been summarized in table 2.4. Note that children less than six months or over six years of age are unlikely to have febrile seizures. If this happens, a thorough investigation should be conducted to determine the underlying cause(s).
Table 2.4 Severe vaccine reactions, onset interval and frequency
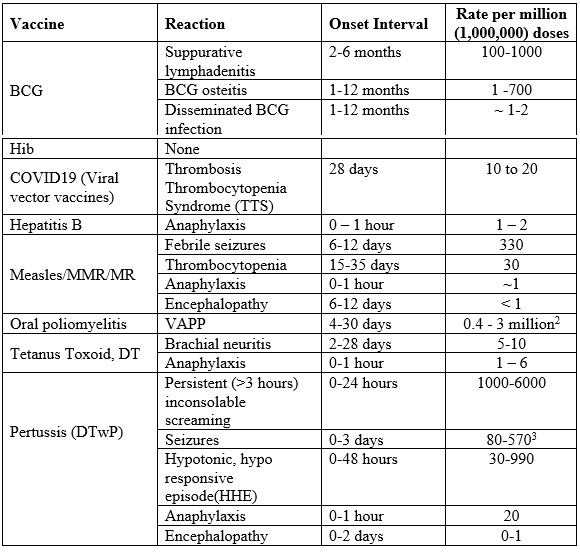
Notes
1. Reactions (except anaphylaxis) do not occur if already immune (~90% of those receiving a second dose are immune): children over six years unlikely to have febrile seizures
2. VAPP Risk is higher following the first dose (1 in 750,000 compared to 1 in 5.1 million for subsequent doses) and for adults and immunocompromised.
3. Seizures are mostly febrile and the risk depends on age, with much lower risk in infants under the age of 4 months.
Immunization error-related reactions
The term “Immunization" as used here means the “use" of a vaccine for the purpose of immunizing individuals. “Use" includes all processes that occur after a vaccine product has left the manufacturing/packaging site – i.e. handling, prescribing and administration of the vaccine.
Immunization error-related reactions are usually preventable and they divert attention from the benefit of the immunization programme. Some of them are described in Table 2.5. The identification and correction of these errors in a timely manner are, therefore, of great importance.
Table 2.5 Immunization error-related reactions
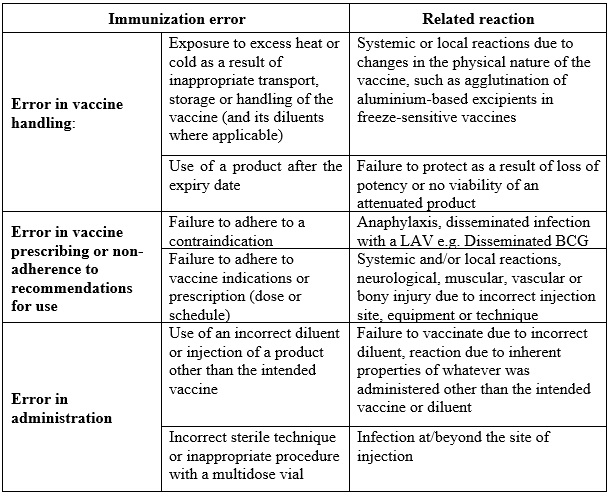
An immunization error-related reaction may sometimes lead to a cluster of events associated with immunization. These clusters are usually linked to a particular provider or health facility, or even to single or multiple vials of vaccine that have been contaminated or inappropriately prepared. For instance, freezing vaccine during transport may lead to an increase in local reactions. The details of an approach to investigating AEFI clusters are described later.
Immunization stress related response (ISRR) formerly known as immunization anxiety related reactions
The term “immunization anxiety-related reaction" was previously used to describe a range of symptoms and signs that may arise around immunization that are related to “anxiety" and not to the vaccine product, a defect in the quality of the vaccine or an error of the immunization programme. These reactions are described as AEFI arising from anxiety about immunization and include vasovagal-mediated reactions (eg fainting), hyperventilation-mediated reactions and stress-related psychiatric reactions or disorders.
The term “anxiety" does not, however, adequately cover the presentation of all these AEFI and anxiety may not manifest during such events. The term ISRR is used to cover the entire spectrum of manifestations (symptoms and signs) of a stress response rather than a single symptom, anxiety. Individual responses to stress vary from person to person or may change according to time or context. In this cause-specific definition, stress results from the process of immunization. As for other AEFI, symptoms may occur during or after immunization; however, in contrast to other AEFI, the symptoms of an ISRR may also occur immediately before immunization.
When ISRR occur in a cluster, they may generate public concern, and, if they are linked to immunization, they may halt or undermine the immunization programme. Although the vaccine is not the underlying cause, the event may be wrongly attributed to it. Halting an immunization programme in such situations will give the impression that the vaccine or the programme is the cause. Various terms have been used to describe such “outbreaks", including “mass hysteria", “epidemic hysteria" and “mass psychogenic illness" ISRR may spread by direct contact and via social media.
Coincidental events
An event may occur coincidentally with immunization and sometimes be falsely attributed to the vaccine i.e. a chance temporal association is falsely attributed to immunization. Such temporal associations are inevitable especially in a mass immunization campaign.
Vaccines are normally administered early in life when infections and other illnesses are common, including manifestations of underlying congenital or neurological conditions. It is, therefore, possible to encounter many events, including deaths that can be falsely attributed to vaccine through a chance association.
For example, incidence of sudden infant death syndrome (SIDS or “cot death") peaks around the age of early childhood immunization. Consequently, many SIDS cases will occur in children who have recently been immunized. However, several well designed studies have shown that the association of SIDS and immunization is coincidental and not causal.
Coincidental adverse events may be predictable. The number of events to be expected depends upon the size of the population and the incidence of disease or death in the community. Knowledge of these background rates of disease and deaths, particularly age-specific disease incidence rates, allows estimation of the expected numbers of coincidental events.
A calculation is shown in Table 2.6 relating to the incidence of infant (under one year) deaths in selected countries to the number of deaths temporally associated with routine DTP or pentavalent vaccine (PVV) immunization. As shown, infant mortality rates result in coincidental deaths in the day, week and month after immunization which are only temporally related to immunization. The actual number of coincidental deaths depends on the population size and infant mortality rate.
Table 2.6 Estimated numbers of coincidental infant deaths that could be temporally linked to immunization (for example with DPT/PVV) in the month, week and day after immunization in some selected countries
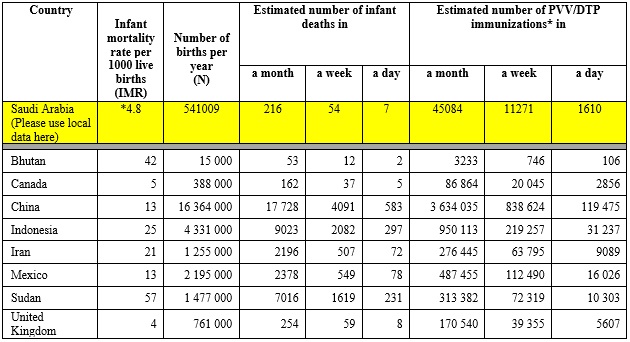
*http://www.stats.gov.sa/en/indicators/55
- General Authority for Statistics –Kingdom of Saudi Arabia
2.2.2 Key AEFI terminology
Cluster of AEFI
A cluster is defined as two or more cases of the same or similar event, which is related in time and has occurred within the same district or geographical unit or associated with the same vaccine, same batch number administered or same vaccinator.
Signal
Information that arises from one or multiple sources which suggests a new potentially causal association or a new aspect of a known association, between an intervention and an event or set of related events, either adverse or beneficial, that is judged to be of sufficient likelihood to justify verificatory action.
Adverse Event of Special Interest (AESI)
An adverse event of special interest (serious or non-serious) is one of scientific and medical concern specific to a particular product or programme, for which ongoing monitoring and rapid communication to the relevant authority (eg regulators) is needed. Such an event may require further investigation in order to characterize and understand it.
An AESI is a pre-specified medically significant event that has the potential to be causally associated with a vaccine product that needs to be carefully monitored and confirmed by further special studies.