7.1 Why we report medication error?
To improve the quality and enhance the safety of patient care.
To prevent errors that have occurred from reoccurring.
Provides best practices for prevention of medication error in health care institutions and develop policies and procedures for medication error prevention
Sources of information for the generation of proactive preventive strategies and best practices aimed toward medication error prevention.
7.2 When we should be report medication error?
Medication Errors that Considered Product-Relate:
Look-alike or Sound-alike similarity between different products names
Look-alike products packaging
Design similarity between two or more products from the same company
Unclear labels or poorly designed packaging including circumstances when wrong or misleading information is presented on outer packaging or inner packaging of medicinal products.
7.3 Who can report medication error?
Consumers, patients, and healthcare professionals can utilize the different channels offered by SFDA to submit a medication error report.
7.4 How to report medication error
Participate in maintaining patient safety through reporting using the suitable reporting tools
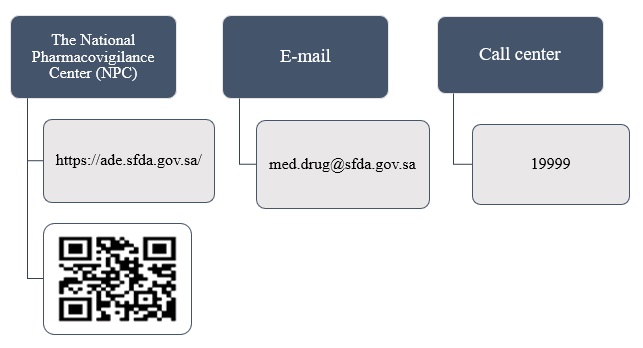
Happens to Submitted Medication Error Reports?
Medication error reports received via any aforementioned channels are logged into SFDA’s databases then investigated to determine registration status as well as cause and contributing factors related to medicinal products. Then, assigned Qualified Person Responsible for Pharmacovigilance (QPPV) of involved product manufacturer is contacted with specific recommendations according to submitted medication error event. Other remedial actions include informing healthcare providers and patients through Risk Minimization Measures published on SFDA website; Saudi Drug Updates (SDU) publications; and use of official circulars.
Report submitter might be contacted, in some cases, if any further clarification or verification of submitted information is required.
7.6 How to register in “Saudi Vigilance System?
Registration in the "Saudi Vigilance System" would save the reporter time and effort while making data entry more accessible due to enrollment.
As a result, the system would retrieve the registered information, and the reporter will not have to input it again.
7.7 Where to find the link for the service?
7.7.1. Direct link ( https://ade.sfda.gov.sa/ )
7.7.2. OR go to |Saudi Food and Drug Authority (sfda.gov.sa) (SFDA’s website)
Click on E-services top menu.
Click on the drug option.
Choose the “Saudi Vigilance System”.
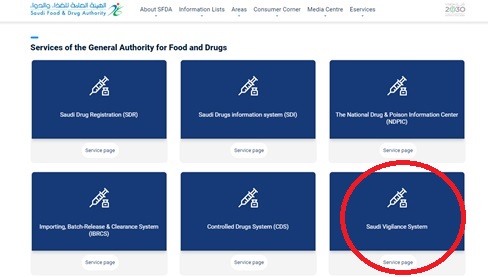
7.7.3. OR google ADE SFDA
7.8 For organizations
7.8.1. Click the “Register” button on the top of the home page
7.8.2. Select “Register Organization”

7.8.3. Complete the register information, attach the nomination letter, then click “Save”
7.9 For individual user registration
7.9.1. Click the “Register” button on the top of the home page
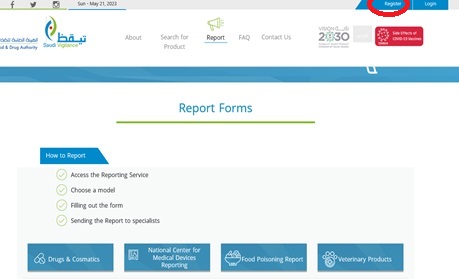
7.9.2. Select “register individual”

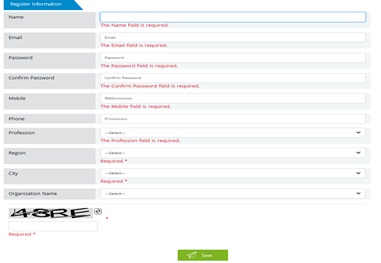
7.9.3. Complete the register information then click “Save”
7.10 For healthcare user registration:
7.10.1. Click the “Register” button on the top of the home page
7.10.2. Select “register healthcare”
7.10.3. Complete the register information then click “Save”