Contraception is categorized as highly effective or effective method based on their failure rates in typical use in the first year. ‘Typical use’ includes user error (for example, missed pills, starting a pack late) or use in circumstances that decrease efficacy such as interactions with concomitant medicines.
Highly effective methods include Copper intrauterine device (copper IUD), Levonorgestrel- Intrauterine delivery system, and Progestogen Implant. The typical-use failure rates are less than 1% and include male or female sterilization.
Effective methods include as following: Progestogen-only injections, which have typical use failure rate of 6%; Combined hormonal contraceptive (pills, patches, or vaginal rings) and progestogen-only pills, which have typical-use failure rates of 9%.
Methods (Natural contraceptive) which used at time of sexual intercourse or based on fertility awareness have higher typical-use failure rates and are not classed as ‘effective’ for use with medicines with teratogenic potential so should not be relied upon alone.
Pregnancy risk should be assessed prior treatment with medicine of teratogenic potential.
• Risk of pregnancy may be high at start of a method or when switching between methods due to risk of pregnancy from unprotected sex prior to starting the method, unreliable use of the previous contraceptive method, and/or time needed to establish contraceptive efficacy at the start of the new method.
• Pregnancy tests at start of contraceptive method may not detect an early pregnancy following unprotected sex in the last three weeks.
Any starter on new method contraception should have a repeat pregnancy test at 3 weeks if there is any risk of pregnancy at start of contraceptive method.
• The duration of teratogen prescriptions may need to be shortened for patients who use contraceptive methods that require frequent pregnancy testing.
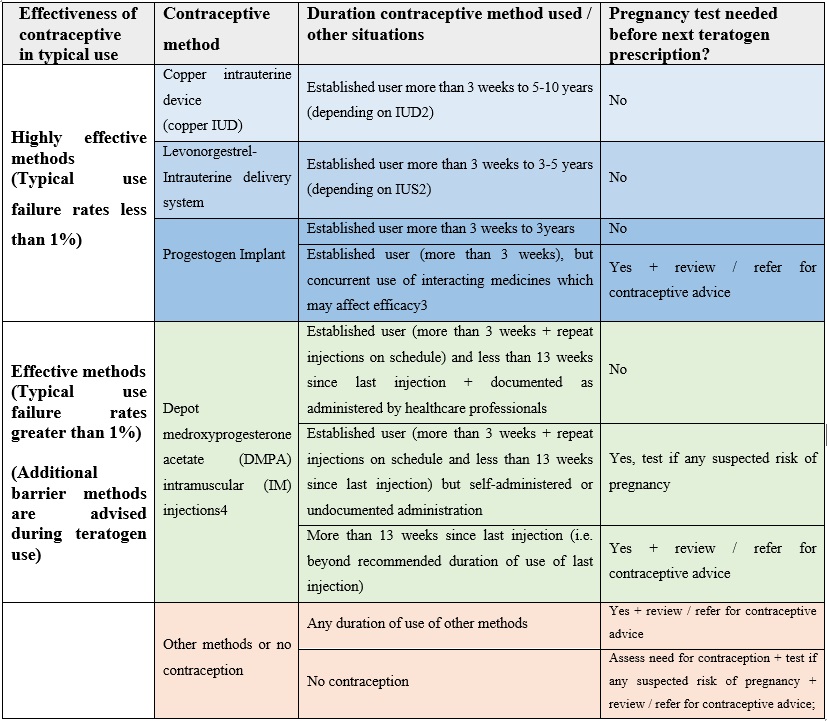
The below table provide guidance to prescribers of medicines with teratogenic potential on the frequency of pregnancy testing needed for most common contraceptive method to avoid exposure in pregnancy during treatment, depending on the chosen contraceptive method. It is recommended to print so can be used as a poster in clinics and to update local guidance, as needed.