For all teratogenic products and for those with pregnancy or breastfeeding related safety concerns in the PSUR, Table 3 below should be provided in the PSUR and filled in completely with reporting period interval and cumulative data. For all other products, reports on pregnancy outcomes in the list below should be provided as available. The congenital malformation rate amongst the exposed is estimated by considering pregnancy exposures at least during the first trimester, collected prospectively and for which the outcome of the pregnancy is known. Additionally, any neonatal adverse reactions and functional anomalies need to be captured. Overall malformation rates as well as the proportional prevalence of individual birth defects have to be compared with relevant reference prevalence rates and discussed, if relevant, by the marketing authorization holder.
Table 3 for reporting numbers of individual case safety reports in PSUR
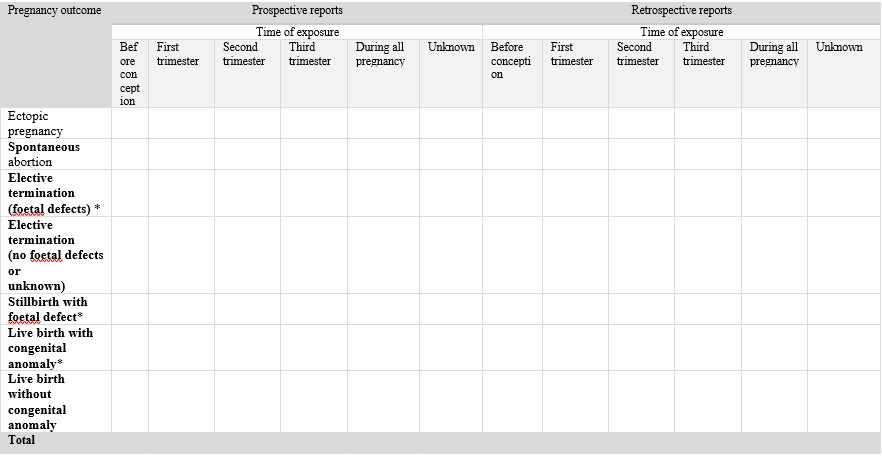
* the observed phenotype should be specified