These requirements applied for topical products listed in Table 1.
Equivalence with respect to quality (extended pharmaceutical equivalence)
Pharmaceutical form, qualitative and quantitative composition, microstructure/physical properties, product performance e.g. dissolution, in vitro release test, and method of administration should be compared.
Product quality equivalence should be undertaken on batches representative of the product to be marketed and the manufacturing process – i.e. batches at production scale. Alternatively, pilot scale batches, at least 1/10 production scale may be used for characterization and comparative purposes, if there are no changes in the manufacturing process and equipment, and evidence provided that scale-up does not affect product quality.
It is acknowledged that there may be only a limited number of representative batches available at the time of submission, and at least three different batches of both the test and comparator products should be compared.
To enable statistical evaluation, the number of samples should be at least 12 units per batch for each experiment.
Extended pharmaceutical equivalence acceptance criteria
The extended pharmaceutical equivalence acceptance criteria between the test and comparator medicinal product are:
A. Pharmaceutical form
The drug product should be the same pharmaceutical form, with the same solution state of the
active substance in the same immiscible phases.
B. Qualitative and Quantitative Composition
- The active substance content and its salt form should be the same.
- In general, the excipients qualitative composition, including grade, if necessary, and quantitative composition of excipients should be the same, although some exceptions are permitted.
In particular, excipients whose function is to influence the active substance solubility, thermodynamic activity or bioavailability and product performance should be qualitatively the same.
The nominal quantitative composition of the excipients should be the same or differences not greater than ±5%. For example, for an excipient present in the comparator medicinal product at 2%w/w, the permitted range in the test product is 1.9 – 2.1%w/w.
- A permitted exception for a qualitatively different excipient may be acceptable for:
• Excipients whose primary function is not related to product performance or administration, i.e. antioxidants, antimicrobial preservatives, colours, and do not have any other functions or effect that influences the active substance solubility, thermodynamic activity or bioavailability and product performance.
Well-established excipients in usual amounts should be employed and possible interactions affecting drug bioavailability and/or solubility characteristics should be considered and discussed.
• Excipient paraffin homologues may be acceptable for excipients whose function relates to the vehicle or emolliency, and do not influence the active substance solubility, thermodynamic activity or bioavailability and product performance.
The different excipient should have no effect on local tolerance or safety. It should be shown that the excipients do not have any other functions or effect that influences the active substance solubility, thermodynamic activity or bioavailability and product performance. In these cases, a biowaiver cannot be justified and is not permitted.
- A permitted exception for a quantitative difference of not greater than ±10% is acceptable:
• For excipients whose function only relates to the vehicle properties or emolliency.
• For excipients whose function is not related to product performance or administration, i.e. antioxidants, antimicrobial preservatives, colours.
It should be shown that the excipients do not have any other functions or effect that influences the active substance solubility, thermodynamic activity or bioavailability and product performance.
C. Acceptance Criteria
- For quantitative quality characteristics, the 90% confidence interval for the difference of means of the test and comparator products should be contained within the acceptance criteria of +/- 10% of the comparator product mean, assuming normal distribution of data.
- Qualitative quality characteristics should be essentially the same.
D. Administration
- The method of administration and administration devices should be similar and achieve the same dose on application.
- If applicable, when product transformation occurs following administration, the test and comparator medicinal product residues are equivalent with respect to quality i.e. in terms of extended pharmaceutical equivalence.
Strength Biowaiver
If several strengths of a test product are applied for, it may be sufficient to establish equivalence at only one strength, which is most sensitive to detect potential differences between formulations.
The following requirements must all be met where a waiver for additional strength(s) is claimed:
a) The different strengths of the test products are manufactured by the same manufacturing process.
b) The different strengths of the test products have the same qualitative composition.
c) The qualitative and quantitative compositions of the different strengths of the test products are equivalent to the different strengths of the comparator medicinal products.
d) Extended pharmaceutical equivalence is demonstrated between the test and comparator medicinal product for all strengths.
Table 1: Specific In vitro requirements for each product:
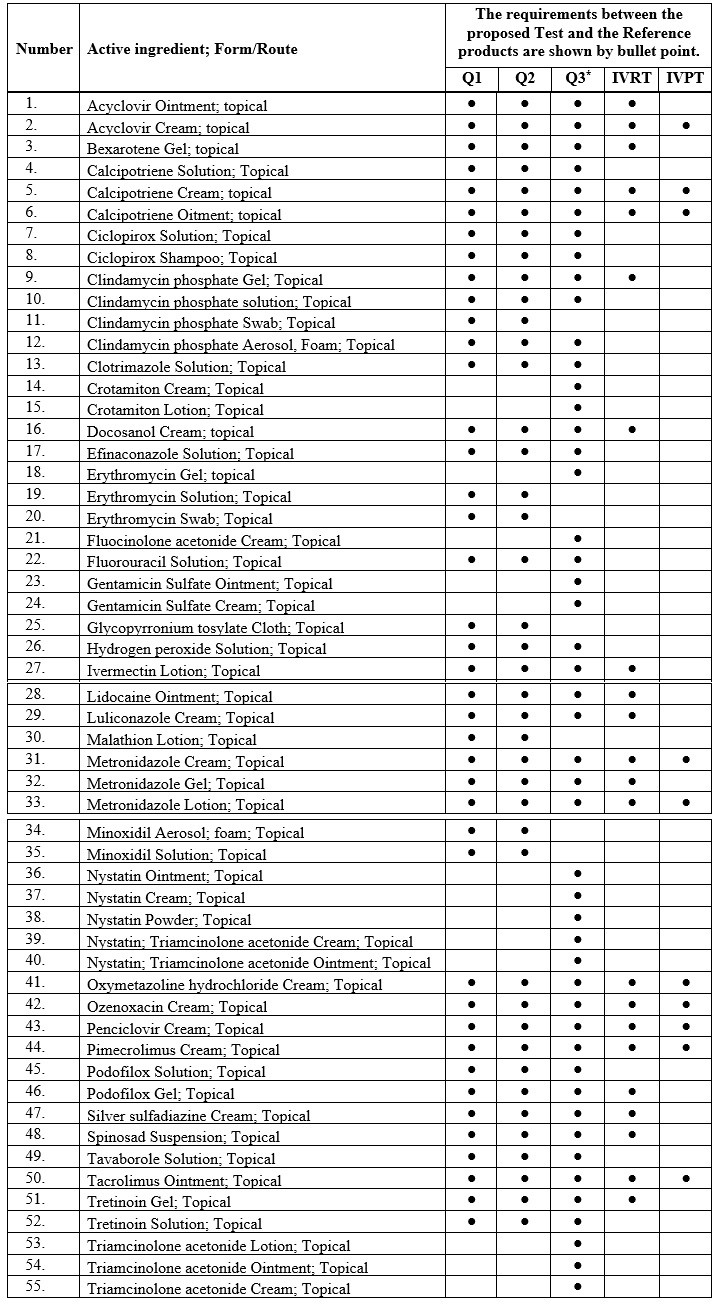
- (*) Follow the comparative physicochemical characterizations test mentioned in the US- FDA Guidance for Physicochemical and Structural (Q3) Characterization of Topical Drug Products.
- There are some exceptions regarding the differences in the quantitative compositions and discussed generally in this guideline.
- For more information on how to perform the in-vitro release test (IVRT) please see Annex 1.
- The waiver of in-vivo requirements may not be accepted when not adhering to the above requirements for the specific product may subject the product to additional requirements.