3.1. Definitions applied to the classification of ATMPs
The following list of criteria is based on the state-of-the-art knowledge and analysis of ATMPs in other regions performed by SFDA. Nevertheless, these criteria should not be considered as exhaustive and might be subject to change as science and technology evolves.
3.1.1. Definition of cell and viable cell for classification purposes
For the purpose of ATMP classification, a cell is defined as follows: ‘A typical cell is the smallest unit of an organism that has been generated directly through mitosis. A cell comprises a nucleus (eukaryotic cells) or nucleoid material (prokaryotic cells) and cytoplasma enclosed by a cell membrane. A viable cell should be capable to produce energy and synthesize new molecules from raw materials.’
A viable cell is a cell that has a functional cytoplasmic membrane. In particular, the concerned method refers to cell staining by viability dyes and manual or automated analysis, under a light microscope or by flow cytometry, of a cell suspension in order to determine the percentage of viable cells.
3.1.2. Claimed Mode of Action (MoA)
Information on the claimed MoA is particularly important to ascertain whether the product is for treatment, prevention or diagnosis of a disease, and exerts its activity via a pharmacological, immunological or metabolic action, or whether the product is intended for regeneration, repair or replacement of cells/tissues. The possible MoA should be considered in relation to the intended indication. For example, if mesenchymal stem cells are used to treat a diseased organ, this could act via a combination of mechanisms which can include metabolic, immunological, pharmacological, regeneration and repair.
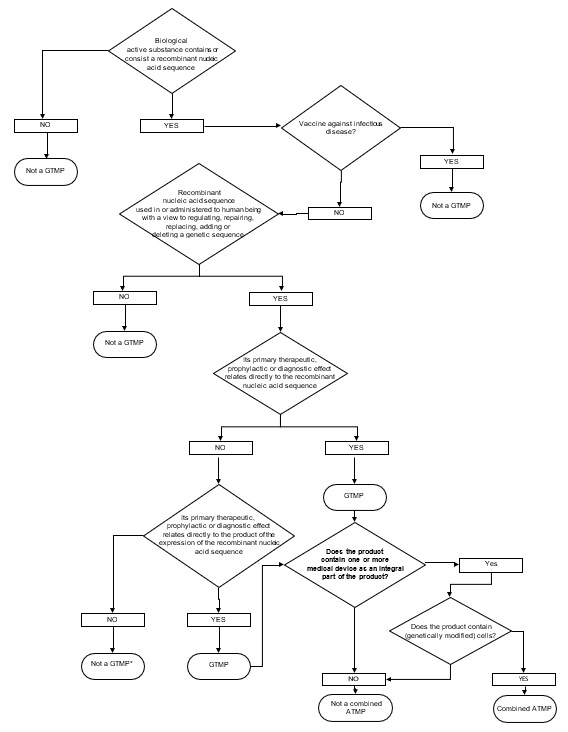
3.1.3. Criteria for GTMP
The definition of gene therapy medicinal product is articulated into two conditions that have both to be fulfilled simultaneously: 1) the product has to be a biological medicinal product and contains recombinant nucleic acid(s) and 2) the recombinant nucleic acid(s) should be directly involved in the mechanism of action (and hence therapeutic action of the product. In this respect, the following observations can be made:
a) of the definition of Gene therapy medicinal product:
The recombinant nucleic acids should be of biological origin independently from the origin of the vector system used (e.g. viral/bacterial vectors or micellar and liposomal formulations, etc.)
b) of the definition of Gene therapy medicinal product:
“its therapeutic, prophylactic or diagnostic effect relates directly to the recombinant nucleic acid sequence it contains, or to the product of genetic expression of this sequence”: The MoA and proposed indication, as claimed by the applicant are of essential to assess if there is a “direct” relationship between the therapeutic, prophylactic or diagnostic effect of the product and the delivered genetic sequence or the expressed product.
Figure 1. DECISION TREE FOR GTMP
3.1.4.Criteria for cell based medicinal products (CBMP)
(Cell based medicinal products include both cell-based product subgroups sCTMP and TEP - both contain or consist of engineered cells or tissues (see definition in ATMP Guideline section 2.1.2. above). To be considered ‘engineered’, cells or tissue(s) should fulfill at least one of the following criteria:
1.Substantial manipulation
Annex I of ATMP Guideline provides detailed description of non-substantial manipulations. The cells or tissue(s) have been manipulated during the manufacturing process so that their biological characteristics, physiological functions or structural properties have been modified to be relevant for their intended function. Examples of substantial manipulations include cell expansion (culture), genetic modification of cells, differentiation/activation with growth factors.
Cell culturing leading to expansion is considered substantial manipulation. Induction of proliferation of cells during cell culture has to be regarded as changes of their biological characteristics and structural properties, either because of an immediate change in cell functionality or cell phenotype, or by increasing cell numbers to augment the desired function of the cells. Furthermore, most adherent cells, for example, are impacted by the repeated attachment and detachment cycles. It has been demonstrated that even the techniques applied for cell detachment might lead to different phenotypic changes especially on cell surface proteins (e.g. membrane receptors).
Enzymatic digestion of a tissue to release cells is also considered to be substantial manipulation, when the aim is to dissociate cell-cell contacts and the released cells are administered into patients with or without subsequent manipulation. An example would be keratinocytes from skin, for which enzymatic digestion would destroy the tissue architecture and functional interactions of the cells, which cannot be regained in the cell suspension: this would be considered as substantial manipulation.
If the enzymatic digestion leads to isolation of functionally intact tissue units (e.g. pancreatic islets and application for Edmonton Protocol) or there is scientific evidence that the original structural and functional characteristics are maintained, the procedure is not considered substantial manipulation.
In case a tissue is treated to remove cells and to be used without any cellular components (e.g., amniotic membrane, bone) the product is not an ATMP because it no longer contains cells or tissues.
If the number of certain cells (e.g., MSCs in fat grafts) is enriched by selection and the processing does not change the characteristics of the cells, this is not considered a substantial manipulation. Additionally, based on scientific considerations, SFDA can also consider other manipulations as “non-substantial”. One example is the radiolabeling of leukocytes for diagnostic purposes. This technique has no significant impact on the functional properties of the cells and should thus not be considered a substantial manipulation.
2.Different essential function (non-homologous use)
Non-substantially manipulated cells or tissues used for the same essential function are not considered ATMPs. In case no substantial manipulation of the cells/tissues takes place, the classification is based on the essential function of the cells/tissues. The same essential function for a cell population means that the cells when removed from their original environment in the human body are used to maintain the original function(s) in the same anatomical or histological environment. Examples of this category are bone marrow cells or peripheral blood cells used for hematopoietic or immune reconstitution. Other clinical uses of bone marrow cells would be considered as ATMPs, unless the same essential function(s) and the same anatomical/histological environment can be demonstrated for the cells/tissues both at the donor and administration site (tissue). The same principle applies to other non-substantially manipulated cells from various origins, for example adipose cells transplanted to other than fat tissue is considered to be ATMPs.
Replacement of a tissue as its whole or functional unit of a tissue (such as cornea or pancreatic islets) is regarded as use for the same essential function. Similarly, transplantation of a non- manipulated tissue to another location in the same anatomical or histological environment is also considered to achieve the same essential function. This is the case for skin transplantation from one part of the body to another part, subcutaneous implantation of pancreatic islets or replacement of arteria by veins. However, in the case of pancreatic islets, the classification will also depend on the manipulation and functional integrity of the islets.
3.Inclusion and exclusions
-Products containing or consisting of animal cells or tissues to be administered to humans will always be considered as ATMPs.
-Products containing or consisting exclusively of non-viable cells or tissues and which do no act principally by pharmacological, immunological or metabolic action, will not be considered ATMPs.
Figure 2. DECISION TREE FOR CBMP (includes sub-groups sCTMP and TEP)
*) see section 3.1.1 on what are considered viable cells. It should be noted that a product containing exclusively non-viable cells/tissue and a medical device / active implantable medical device as an integral part, will be considered a combined ATMP when these non-viable cells/tissues exert the primary action of the combined product. This primary action should be based on the pharmacological, immunological or metabolic action of the non-viable cells/tissues.
3.1.5.Criteria for combined ATMPs
Combined ATMPs incorporate an active substance, i.e. a cellular or tissue part consisting of viable or non-viable cells or tissues and of one or more medical devices or one or more active implantable medical devices as an integral part of the product. The medical device(s) should be used in the combination in the same way as its intended use without additional components. If cells or tissues are not viable, these must exert the primary action of the combined product.
It should be noted that normally the medical device should retain its intended purpose/ mode of action in the combination to be considered as being “integral part” of the final product and thus qualify this product as a combined product. For those products where the function of the matrix is no longer considered to be linked to its structural properties, classification of non-combined ATMP will be applied by SFDA.
3.2.Evolving and borderlines areas
The ATMP classification procedure will also have to clarify borderline cases between ATMPs versus non-ATMPs as well as between the different product categories within the ATMP sphere. Below are given examples that illustrate the type of issues that are taken into consideration when assessing borderline cases.
3.2.1.Advanced therapies versus transplants/transfusion
Products consisting of cells or tissues may be at the border between SFDA Tissues and Cells legislation and the ATMP Guideline. Cells/tissues harvested and separated by a simple selection method (that does not result in a substantial manipulation of the cells/tissue) and re-administered to fulfill their same essential function will generally be regarded as non-ATMPs. However, depending on whether or not the selection process/method will alter the original characteristics of the cells/tissues may result in classification as ATMPs. Similarly, cells derived from human blood (e.g. lymphocytes) that are substantially manipulated or use for a different essential function are classified as ATMPs.
One example is that preparation of human pancreatic Langerhans’ islets (i.e. Edmonton Protocol) should not be classified as an ATMP. It will be considered that, for this preparation, the described process steps do not constitute substantial manipulations for the intended use so that there is no change in the biological characteristics of the islets. In addition, the product was intended to be used for the same essential function in the recipients, be it in the allogeneic or autologous conditions described. This conclusion is, however, not directly applicable to any other pancreatic beta cell products which may be submitted for classification, as they may be derived from very different and more complex process and substantial manipulations, i.e. cell-based product consisting of isolated beta-cells embedded in an alginate matrix.
In contrast, some products with an essentially minimal manipulation or maintenance of the initial biological properties can be classified as ATMP due to their intended use based on (a) different essential function(s) of the cells/tissues. For example, the use of autologous bone marrow-derived progenitor cells intended for treatment of patients with myocardial infarction or other vascular diseases would be considered as different essential function and therefore such products are classified as ATMPs.
It is possible that cell-based products administered in the same anatomical location fall under the definition of ATMP on grounds that it is used for a different essential function. This can be encountered when the mode of action of the cells is not identical to the one attributed to the cells by the scientific knowledge, for example, the injection of concentrated bone marrow at the site of bone injury with the aim of healing a bone lesion.
3.2.2.Gene therapy medicinal product versus cell based medicinal product
Another borderline scenario can relate to products that are modified by adding an mRNA sequence, for example dendritic cells (DC) electroporated with mRNA in vitro and administrated to the patient to elicit a specific immune response. One could argue that the claimed mechanism of action is directly related to the expression of the mRNA encoded antigens to stimulate e.g. tumor specific immune responses. However, due to its relatively short half-life there may be little or no residual mRNA at the time of re-administration of the dendritic cells to the patient. Thus, it can be claimed that a recombinant nucleic acid is not administered to human beings with a view to adding a genetic sequence, but rather the mRNA electroporated DCs could be seen as an intermediate in the manufacturing process where the phenotype is finally altered without alteration of the genotype of the cells. Therefore, the product was considered not to comply with the definition of a gene therapy medicinal product. Instead, this ATMP Guideline considers that the product is a cell-based therapy product as it consists of cells that were administered to human beings with a view to treating a disease through the immunological action of the modified cell populations.